Emerging Contaminants in Water: Uncovering Environmental Crimes
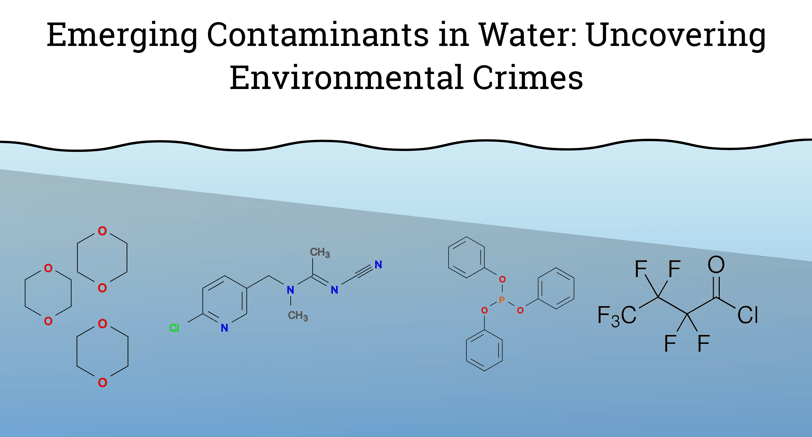
Understanding the chemistry between water and contaminants is crucial for addressing environmental pollution and ensuring safe drinking water. Water's polarity and hydrogen bonding capabilities make it an exceptional solvent. The polar nature of water molecules, with a partial negative charge near the oxygen atom and a partial positive charge near the hydrogen atoms, allows water to dissolve ionic compounds and polar molecules effectively. Chemical contaminants are an ever-evolving concern due to new discoveries of substances and their effects on health and the environment. Here are some examples of chemical contaminants interactions with water:
Per and Polyfluoroalkyl Substances (PFAS): PFAS are characterized by their carbon-fluorine bonds, which are among the strongest in organic chemistry. This bond strength imparts remarkable stability and resistance to degradation, earning PFAS the nickname "forever chemicals." Common examples include perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS). PFAS molecules have a hydrophobic tail and a hydrophilic head, allowing them to form micelles in aqueous environments. This amphiphilic nature enhances their mobility in water, facilitating widespread distribution in groundwater and surface water bodies. The unique properties of PFAS, such as hydrophobicity and lipophobicity, arise from the fluorinated carbon chain, which repels both water and oil. This makes PFAS ideal for applications requiring water and stain resistance, such as in non-stick cookware, waterproof fabrics, and firefighting foams. However, these same properties contribute to their environmental persistence, and bioaccumulation, has been linked to adverse health effects such as cancer, liver damage, immune system disruption, and developmental issues in infants and children. In response to growing concerns about PFAS contamination, the U.S. Environmental Protection Agency (EPA) has established health advisory levels of 70 ng/L for PFOA and PFOS and is working on comprehensive regulations for a broader range of PFAS compounds.
Neonicotinoid Pesticides: Neonicotinoid pesticides are a class of neuro-active insecticides chemically similar to nicotine. These compounds are widely used in agriculture to protect crops from various pests. However, their widespread use has raised environmental concerns, particularly regarding their impact on water systems and non-target organisms such as bees. Neonicotinoids typically have a chemical structure characterized by a nitro or cyano group attached to a heterocyclic ring. Examples include imidacloprid, clothianidin, thiamethoxam, and acetamiprid. These compounds are generally water-soluble, due to the presence of polar functional groups like amines and nitrogen in their structure. While it makes them effective for treating plant tissues, it also means they can easily move through the environment. The interaction between neonicotinoids, water, and other contaminants creates a complex system that can affect the persistence and movement of these insecticides in the environment. This can potentially lead to unintended consequences for aquatic life and water quality.
1,4-Dioxane: It mixes incredibly well with water, like sugar dissolving in your coffee. This is because 1,4-Dioxane is a cyclic ether molecule with polar functional groups, particularly oxygen atoms. These oxygen atoms are the key players. They can form hydrogen bonds with water molecules. Hydrogen bonding is like a tiny attraction between molecules, and in this case, it allows 1,4-Dioxane to integrate seamlessly with water. A major historical use was as a stabilizer for solvents like trichloroethane (TCA) used in metal degreasing. The U.S. Environmental Protection Agency (EPA) is currently evaluating the risks of 1,4-Dioxane to human health and the environment.
Organophosphate Flame Retardants (OPFRs): Organophosphate flame retardants (OPFRs) are a class of chemicals used to make materials more fire-resistant. The presence of a phosphate group can contribute to water solubility. The phosphate group has charged regions that can interact with water molecules through ionic interactions. Attached to the phosphate group are organic chains (alkyl or aryl groups). The length and chemical nature of these chains can significantly impact solubility. Shorter, more polar chains tend to increase water solubility, while longer, non-polar chains decrease it. Some countries, like Sweden and Denmark, have completely banned certain OPFRs due to environmental and health concerns.
Water’s ability to dissolve a wide range of substances, while beneficial in many contexts, also means it can carry contaminants over long distances and into drinking water supplies. Understanding the interactions between water and chemical contaminants such as PFAS, neonicotinoids, 1,4-Dioxane, and OPFRs is crucial for developing effective strategies to mitigate pollution and protect environmental and human health. Regulatory measures, advanced treatment technologies, and ongoing research are essential components in addressing the challenges posed by these persistent contaminants.
